Chapter 5 The Greenhouse Effect
In this chapter, I will describe the processes that control the temperature of the Earth.
5.1 The Goldilocks Paradox
The Earth’s temperature has varied over time throughout Earth’s history, but the range of variation has been remarkably small. Earth is unique among all the planets in the solar system because it has liquid water. Earth and its nearest neighbors, Mars and Venus, form the three terrestrial planets: planets of similar size, whose distances from the sun put them in a narrow band called the continuously habitable zone. All three planets are large enough for their gravity to maintain permanent atmospheres that will not escape to space, but not so large that the gravity will crush soft objects such as living beings. The continuously habitable zone, in which all three planets lie, is close enough to the sun for sunlight to melt ice so liquid water can form, but not so close that the sunlight will boil all the water away, as it would on the planet mercury.
Thus all three planets could, in principle, have bodies of liquid water on their surfaces and could thus support life. Indeed, scientists believe that at one time, a few billion years ago, all three planets probably did have large seas. However, today only the Earth has liquid water on its surface. Mars is frozen solid, so cold that even the carbon dioxide in its atmosphere has mostly frozen and fallen to the surface as dry ice. Venus lies at the other extreme. Its surface is hot enough to melt lead and all of its water boiled away long ago.
The Michael Rampino and Ken Caldeira described this as the “Goldilocks problem:” Why did three planets, of similar size and located at similar distances from the sun, produce such different conditions, with Mars far too cold to support liquid water and life, Venus far too hot, and the Earth just right, like the three bears’ bowls of porridge in the fairy tale? (Rampino & Caldeira 1994) The answer to the Goldilocks problem will also explain why burning fossil fuels is causing our planet to warm up.
5.2 The Temperature of Planets
The first step in understanding the different fates of the terrestrial planets is to remember the fundamental principle of planetary temperature: All planets receive heat from the sun and emit heat to outer space. A planet’s temperature is constant when the amount of heat coming in from the sun balances the heat going away to space. If more heat comes in from the sun, then the temperature will keep rising and the planet will grow hotter and hotter. If more heat is leaving to space, then the temperature will keep dropping, and the planet will get colder and colder.
Although the Earth’s temperature has varied over time, geologists have found extensive evidence that there has always been liquid water on the surface for at least the last three and a half billion years. This means that the amount of heat coming in from the sun has balanced the heat going out to space very closely over all of that time. Small imbalances have occurred, which have caused temperature variations over Earth’s history, such as the Pleistocene ice ages that came and went over the last 2.75 million years, burying much of what is now the Northern United States and Northern Europe under thousands of feet of ice; or the warm Cretaceous, when there was no ice in the Arctic, the Arctic ocean was full of seaweed, and tropical breadfruit grew in Canada. But throughout all of those temperature changes, there were always liquid oceans and life continued to thrive. This would not have been possible if the imbalances between incoming and outgoing heat grew large.
It would be almost inconceivable for the Earth to consistently achieve this close balance between incoming and outgoing heat for billions of years if it happened purely by chance. But chance had little to do with it. It turns out that there is are mechanisms in the Earth system that act like thermostats to control the temperature by actively maintaining the balance between incoming and outgoing heat.
5.2.1 Radiation and Heat
Heat can flow through many different mechanisms. When you put a kettle on the stove to boil water, the outside of the bottom of the kettle is heated by the burner. This heat flows through the metal body of the kettle to the inside by conduction. The water at the bottom of the kettle is then heated by contact with the kettle. The warm water at the bottom rises and mixes with the rest of the water so all of the water heats up. This is convection. Convection is also how the heating and cooling systems in your house or your dorm room manage to control the temperature of the entire room by sending warm or cool air through vents in the floor or ceiling.
But the Earth sits in the vacuum of space. Neither convection nor conduction work in a vacuum. Indeed, many of the best insulated containers for keeping drinks hot or cold work by having two layers of metal with vacuum in between them.
However, we all know that heat can cross 93 million miles of vacuum to get from the sun to the Earth. We can feel that heat on our bodies when we sit in the sunlight. Indeed, a third important way that heat can flow from hot things to cold things is by electromagnetic radiation.
There are many kinds of electromagnetic radiation. We are most familiar with visible light, but ultraviolet light, gamma rays, infrared light, microwaves, and radio waves are other forms of electromagnetic radiation.
The two characteristics of electromagnetic radiation that we will need to know about for this class are its intensity and its wavelength. The intensity is basically a measure of how bright the radiation is. When you see a bright light, its intensity is large. When you see a dim light, its intensity is small.
The wavelength is similar to color. With visible light, the wavelength of the light is what causes your eyes to see color. Red light has longer wavelengths, green and yellow light has intermediate wavelengths, and blue and violet light has short wavelengths. Your eye can see a range of wavelengths equivalent to one musical octave.
Ultraviolet light, X-rays, and gamma rays are electromagnetic radiation with wavelengths too small to see. Infrared light, microwaves, and radio waves are electromagnetic radiation with wavelengths too long to see.
There are six principles about wavelength and intensity of electromagnetic radiation that you need to know to understand how the climate system works:
Everything—solid, liquid, or gas—is constantly emitting electromagnetic radiation.
The hotter something is, the more intense the radiation it emits.
THe hotter something is, the shorter the wavelengths of the radiation it emits.
Everything absorbs electromagnetic radiation to some degree, but different things absorb the different wavelengths differently.
In gases, each molecule absorbs certain wavelengths and does not absorb others.
Things that absorb well at a certain wavelength also emit well at that wavelength.
You have probably experienced some of these principles in your life, although you may not have thought about them in scientific terms:
- Brighter light bulbs tend to get hotter.
- Fires that give off intense heat tend to have brighter flames.
- If you have an electric stove at home, or a quartz space heater, you may have noticed that when the burner of the stove or the heating rods of the space heater get hot, they start to glow red, then orange at hotter temperatures, and finally, yellow or white when they get extremely hot.
- Contactless thermometers, such as the ones you stick in your ear, work by measuring the intensity of infrared light emitted by your eardrum. If you are running a fever, your core body temperature is higher and the infrared light from your eardrum is more intense.


Figure 5.1: My pet dog Finley, as seen by a camera at visible wavelengths and at infrared wavelengths.
Figure 5.1 shows my dog Finley seen by a camera that can sense both visible light and infrared light. The image on the left shows reflected from lights in the room. The colors correspond to the wavelengths of light reflected by the different objects. The image on the right shows the infrared light emitted by Finley’s body. The colors are purple and blue where where the infrared light intensity is small (colder temperatures) and orange or yellow where the intensity is high (hotter temperatures). The tile floor is fairly cold, so it shows up as dark purple. Finley’s body is hot (especially his ear), so that shows up as yellow with the hottest part (his ear) in white. During the COVID pandemic, public health experts are experimenting with using this kind of infrared camera in public places, such as airports and the entrances to buildings, to detect people with fevers,
5.2.2 The Sun and the Earth
The surface of the sun is about 5,500 Kelvin (9,400°F) and the surface of the Earth is, on average, about 290 Kelvin (63°F). The sun is almost 20 times hotter than the Earth, so the sun emits visible light over a broad range of wavelengths that cover the entire visible region of the spectrum. Sunlight is most intense at a wavelength of about 0.5 microns (\(\mu\)m),4 which corresponds roughly to the color green, but the sun looks white or yellow, rather than green, because your eyes see the whole range of wavelengths it emits. The Earth is much colder, so it emits radiation that is much lower intensity and much longer wavelength. The radiation emitted by the Earth covers a broad range of wavelengths in the infrared, and is most intense at a wavelength around 10 microns, about 20 times longer than sunlight wavelengths.
So the Earth is constantly absorbing heat from the sunlight and simultaneously, it is emitting heat that goes out into space.
Radiation becomes less intense as you move away from the source. You have experienced this if you have been near a hot fire in a fireplace or a bonfire and found that the heat from the fire feels more intense as you move closer and less intense as you move farther away.
The sun is 93 million miles away from the Earth, so by the time sunlight gets to us, it is about 50,000 times less intense than it is at the surface of the sun. This is good because the intensity near the surface of something that’s 9,400°F is enough to incinerate a person.
I won’t go into the detailed math here, but when we consider the way that intensity depends on temperature and the fact that the sun only shines on half of the Earth, and the fact that light-colored things on the Earth, such as clouds, snow, and ice, reflect sunlight, scientists calculate that the heat the Earth absorbs from the sun is equal to the heat the Earth emits to outer space.
5.3 Earth’s Temperature: Balancing the Flow of Heat
It is not a coincidence that the heat the Earth absorbs from sunlight equals the heat it emits to space. The intensity of heat the Earth emits as infrared light depends on the temperature. The intensity is greater if the Earth is hotter and smaller if the Earth is cooler.
Let’s consider what happens if the absorption and emission are out of balance. Suppose the Earth is absorbing more heat from sunlight than it is emitting to space. Our principle of balance tells us that this will cause the temperature to rise. And as the temperature rises, the Earth emits more heat to space. Eventually, the temperature will rise enough for the emitted heat to equal the absorbed heat and then the temperature will stop changing.
If the Earth is emitting more heat than it is absorbing, the opposite happens: the principle of balance tells us that the temperature will drop, which will then reduce the intensity of emitted heat. Eventually the temperature will drop enough so the emitted heat balances the absorbed heat and then the temperature will stop changing.
Thus, whichever way the Earth is out of balance, the imbalance of heat coming in and going out will cause Earth’s temperature to change in a way that brings it back into balance. In this way, thermal radiation automatically keeps the incoming and outgoing heat in balance.
5.4 The Greenhouse Effect
The greenhouse effect is a consequence of the principles of radiant heat that I described above:
Hotter objects radiate at shorter wavelengths.
The sun is 20 times hotter than the Earth, so its radiation is mostly visible light, where the Earth’s is infrared, with much longer wavelengths.
Different molecules absorb different wavelengths.
The atmosphere is transparent to visible light. We can see through about 100 miles of air to see the sun, the moon, and the stars. However, carbon dioxide and water vapor absorb infrared light at the many of the same wavelengths that the Earth radiates. This means that instead of escaping to space and taking heat away from the Earth, most of the infrared light emitted by the surface gets absorbed by CO2 and H2O molecules and heats the atmosphere instead.
5.4.1 A Simple Explanation of the Greenhouse Effect
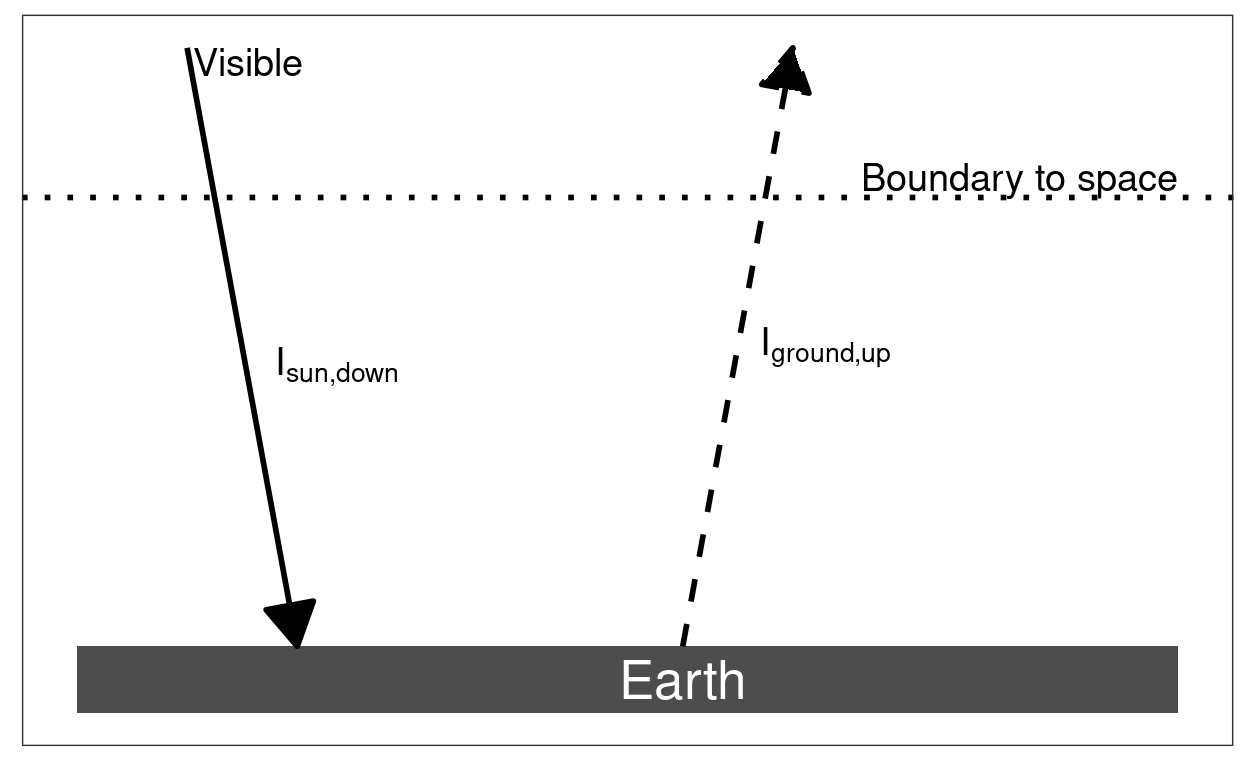
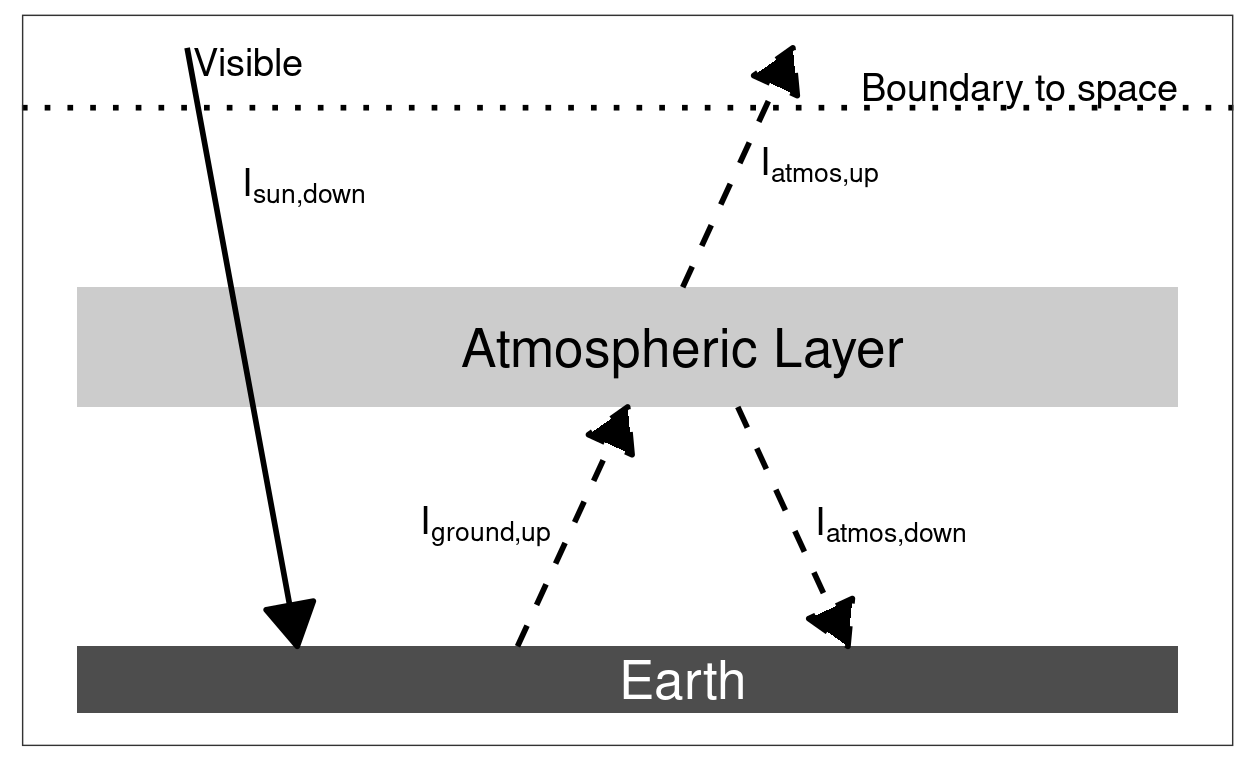
Figure 5.2: Heat flow and the greenhouse effect. Without the greenhouse effect, visible light from the sun brings heat to the Earth’s surface and infrared light emitted by the surface carries heat away to space. With a greenhouse effect, greenhouse gases in the atmosphere are transparent to visible light from the sun, but absorb the infrared light from the surface before it can escape to space. This absorbed heat warms the atmosphere, which then emits heat upward, carrying heat to space, and downward, where it adds to the heat the surface receives from the sunlight.
The basic principles of the greenhouse effect are illustrated in Figure 5.2. The figure shows visible sunlight bringing heat to the Earth and infrared light sending heat out to space. The figure also shows an imaginary boundary between the atmosphere and outer space. There is not really a sharp boundary—the atmosphere gradually fades away rather than just stopping at some height—but if we imagine a clear boundary, it makes things simpler. If we look at the flow of heat at this boundary, the Earth’s temperature will be steady if and only if the heat coming in (the visible light from the sun) matches the heat going out (infrared light going to space). The intensity of the incoming sunlight is the same, regardless what the Earth’s temperature is, but the intensity of the outgoing infrared light depends on the Earth’s temperature. Thus, the temperature adjusts until the outgoing infrared light matches the incoming visible sunlight.
The left panel of the figure shows the situation if there is no greenhouse effect. This could represent the Earth with no atmosphere, or with an atmosphere that contains no greenhouse gases (carbon dioxide, water vapor, etc.). Here, the surface heats up until the infrared light it emits (\(I_{\text{ground}, \text{up}}\)) matches the visible light coming from the sun (\(I_{\text{sun}, \text{down}}\)). If we worked through the detailed equations of heat flow, this temperature turns out to be approximately 255 Kelvin, or about 0°F.
The right panel of the figure shows how the flow of heat changes if there is a strong greenhouse effect. Here, we imagine that the amount of greenhouse gases in the atmosphere is so great that they absorb 100% of the infrared light emitted by the surface. We also imagine that the atmosphere is a uniform and homogeneous slab of air.
This is a gross oversimplification of the real atmosphere, which varies considerably from place to place (humid over the oceans, dry over deserts, thick near sea-level and thin at high altitudes), and which absorbs most infrared light from the surface, but not all of it. This simplification makes it much easier to study the big picture of the greenhouse effect without becoming distracted by details and it produces a surprisingly good estimate of the actual greenhouse effect.
If the atmosphere absorbs all of the infrared light coming from the surface, then how does heat escape to space to balance the heat flow at the imaginary boundary above the top of the atmosphere? When the atmosphere absorbs heat from the surface, it heats up until it emits enough infrared light to balance its own heat flow (the heat absorbed by the atmosphere must equal the heat emitted from the atmosphere in order for the atmosphere to have a steady temperature). This means that the atmosphere has to be hot enough that the top of the atmosphere emits as much heat to space as the sun’s visible light brings to the Earth.
However, the atmosphere does not only emit light going up. It also emits light downward toward the surface. Here, we assume that the atmosphere has the same temperature from top to bottom (this is another oversimplification). This allows us to work out the heat flow easily:
- The intensity of heat going up from the top of the atmosphere as infrared light (\(I_{\text{atmos}, \text{up}}\)) must equal the amount of heat coming in as visible sunlight (\(I_{\text{sun}, \text{down}}\)): \[I_{\text{atmos}, \text{up}} = I_{\text{sun}, \text{down}}.\]
- The intensity of radiant heat emitted by an object (the atmosphere, the surface of the Earth, the surface of the sun, etc.) depends only on the temperature of the object. This means that if the bottom of the atmosphere is the same temperature as the top, then the intensity of the heat going down from the atmosphere to the surface (\(I_{\text{atmos}, \text{down}}\)) must be equal to the intensity of heat going up to space (\(I_{\text{atmos}, \text{up}}\)), which in turn equals the intensity of visible light coming from the sun (\(I_{\text{sun}, \text{down}}\)): \[ I_{\text{atmos}, \text{down}} = I_{\text{atmos}, \text{up}} = I_{\text{sun}, \text{down}}. \]
- The surface of the Earth is receiving heat both from the sun and from the atmosphere. In this simple picture, the intensity of the heat coming down from the atmosphere (\(I_{\text{atmos}, \text{down}}\)) equals the heat coming from the sun, so the surface receives twice as much heat with the greenhouse effect as it would without a greenhouse effect.
- The temperature of the surface will adjust until the amount of heat it emits equals the amount it absorbs. This means that the heat going up from the surface must be twice the intensity of the incoming sunlight. \[ I_{\text{ground}, \text{up}} = I_{\text{atmos}, \text{down}} + I_{\text{sun}, \text{down}} = 2 I_{\text{sun}, \text{down}} \]
- You can add up the numbers to see that everywhere in the system, they match:
At the boundary to space, the total heat coming into the Earth system from the sun equals the heat going out to space from the top of the atmosphere: \[I_{\text{sun}, \text{down}} = I_{\text{atmos},\text{up}}.\]
At the atmosphere, the heat going into the atmosphere from the surface equals the amount of heat emitted from the atmosphere to space and to the surface: \[ I_{\text{atmos}, \text{down}} + I_{\text{atmos}, \text{up}} = I_{\text{ground}, \text{up}}. \] We don’t count the heat from the sun here (\(I_{\text{sun},\text{down}}\)), because the atmosphere is transparent to sunlight, so the sunlight is not absorbed by the atmosphere, but passes through and gets absorbed by the ground.
This equation gives the detailed explanation of why the heat flow into and out of the atmosphere balance each other: \[ \begin{aligned} I_{\text{atmos}, \text{down}} + I_{\text{atmos}, \text{up}} &= 2 I_{\text{atmos}, \text{up}} \\ &= 2 I_{\text{sun}, \text{down}} \\ &= I_{\text{ground}, \text{up}} \\ \end{aligned} \]
At the surface, the heat going up equals the heat coming down from the sun plus the heat coming down from the atmosphere: \[ I_{\text{ground},\text{up}} = I_{\text{sun},\text{down}} + I_{\text{atmos},\text{down}} = 2 I_{\text{sun},\text{down}} \]
We won’t go into the math of how the temperature relates to the intensity of radiant heat, but if we solve the detailed equations for this simple layer model, it predicts that the temperature of the surface would be 303 Kelvin, or about 86°F. In fact, the average surface temperature of the Earth is about 290 K, or 63°F.
The discrepancy between the prediction and the actual temperature is due to the simplifications we made by assuming that the atmosphere absorbs all of the infrared light from the surface and that the atmosphere is a uniform slab of air with the same properties at every height from the bottom to the top and at every part of the Earth, from the oceans to the deserts and from the tropics to the poles. The more careful and detailed models that scientists use can predict temperatures very accurately and have a good track record of predicting the observed trend of global warming.
This course does not focus on mathematical treatments of the climate, so the thing you should take away from this section is that the greenhouse effect works because of the following properties:
- The atmosphere is transparent to visible light but opaque to infrared light.
This means that
- Sunlight travels through the atmosphere and directly heats the surface.
- Infrared light emitted from the surface is absorbed by the atmosphere.
- When the atmosphere absorbs infrared light from the surface, it heats up until it emits as much heat as it absorbs.
- Infrared light from the atmosphere goes both up to space and down to the surface, so the surface now receives heat both from the sun and from the atmosphere.
The greenhouse effect raises the temperature of the surface by adding a new source of heat to the surface.
The implications for global warming are that the more greenhouse gases, such as CO2, are in the atmosphere, the more of the heat from the surface it absorbs. The more heat the atmosphere absorbs from the surface, the hotter it gets, and the more heat it sends down to the surface. That is a simple, but reasonably accurate, picture of how global warming works.
5.5 Goldilocks and the Greenhouse
The greenhouse effect provides part of the resolution to the Goldilocks paradox.
| Quantity | Earth | Mars | Venus |
|---|---|---|---|
| Intensity of sunlight | 1,350 \(W/m^2\) | 600 \(W/m^2\) | 2,604 \(W/m^2\) |
| Surface temperature | 295 K = 71° F | 240 K = -28° F | 700 K = 800° F |
| without greenhouse | 254 K = -2° F | 216 K = -70° F | 240 K = -27° F |
| Greenhouse effect | 41 K = 74° F | 24 K = 42° F | 460 K = 828° F |
| Atmospheric pressure | 1,013 millibar | 6 millibar | 92,000 millibar |
The reason for the three planets’ different temperatures is the very different magnitudes of their greenhouse effect. Mars’s atmosphere is very thin—it has less than one percent as much air as the Earth—and not only is Venus’s atmosphere almost 100 times as massive as the Earth’s, but its atmosphere is almost pure carbon dioxide, so it has about 230,000 times more CO2.
The second part of the mystery of the Goldilocks problem is how the planets ended up with such different atmospheres. This will have to wait for the next chapter, where we will study the biogeochemical carbon cycle that controls the amount of carbon dioxide in the atmosphere.
We will see that the planets probably started out with similar atmospheres, but small differences among them led Mars to lose almost all of its atmosphere and Venus to experience a runaway growth of carbon dioxide, while the Earth’s atmosphere remained very stable.
References
Rampino, M. R., & Caldeira, K. (1994). The Goldilocks problem: Climatic evolution and long-term habitability of terrestrial planets. Annual Review of Astronomy and Astrophysics, 32, 83–114.
A micron, or \(\mu\)m, is one one-millionth of a meter, or about one tenth the size of a red blood cell.↩︎